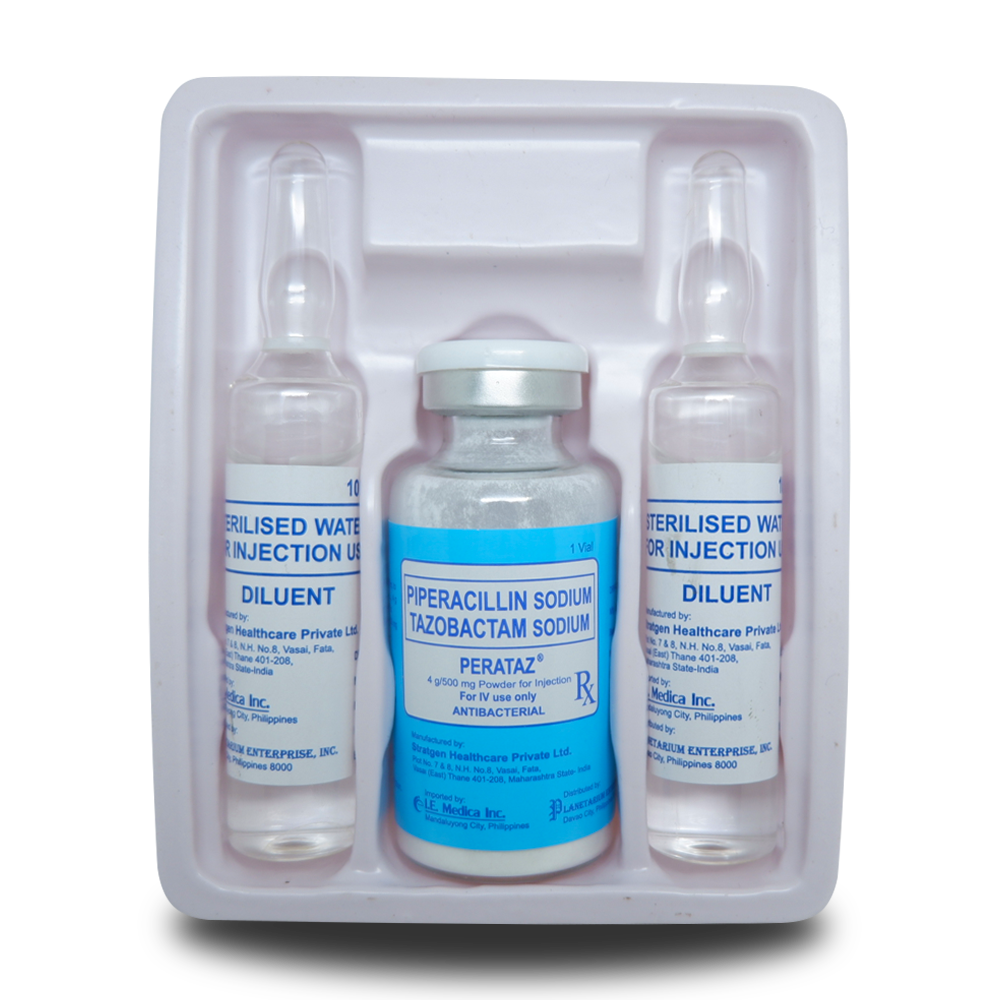

Generic Name: PIPERACILLIN SODIUM TAZOBACTAM SODIUM
Perataz®
4 g/500 mg Powder for Injection
For IV use only
ANTIBACTERIAL
Formulation:
Each vial contains:
Piperacillin Sodium USP eq. to PiperacilHn ……………. 4 g
Tazobactam Sodium eq. to Tazobactam ………………… 500 mg
Indications:
In adults and children over 12 years of age:
In children under 12 years of age:
Pharmacodynamics
Piperacillin is an antibiotic that has the ability to kma wide variety of bacteria. It works by interfering with the formation of bacterial cell walls. It does this by preventing the bacteria forming vital cross-links within their cell walls. These cross-links strengthen the cell walls and allow them to protect the bacteria from their environment. By interfering with the cross-linking meshwork in the cell walls, Piperacillin weakens them.
The cell walls of bacteria are essential for their survival. They protect the bacteria from the environment and keep unwanted substances from entering the cells. As Piperacillin weakens the cell walls, it allows unwanted sllbstances to enter the bacterial cells. This causes the cells to swell and eventually rupture, and kills the bacteria.
Certain bacteria are resistant to penicillin-type antibiotics, because they have developed the ability to produce defensive chemicals. These chemicals are called beta-lactamases. They interfere· with tlie structure of penicillin-type antibiotics and stop them from working.
Tazobactam is a type of medicine known as a beta-lactamase inhibitor. It is included in this medicine because it inhibits the action of the beta-lactamases produced by in defense by certain bactria. This prevents the bact3ria form inactivating the Piperacillin, and leaves them susceptible to attack. Tazobactam therefore increases the range of bacteria that Piperacillin can kill.
Pharmacokinetics
After the administration of single doses of PiperaCillinrrazobactam to subjects with renal impairment, the half-life of Piperacillin and of Tazobactam increases with decreasing creatinine clearance. At creatinine clearance below 20 mUmin, the increase in half-life is twofold for Piperacillin and fourfold forTazobactam compared to subjects with normal renal function. Piperacillin and Tazobactam are widely distributed int tissues and body fluids including intestinal mucosa, gallbladder, lung, female reproductive tissues (uterus, ovary, and fallopian tube), interstitial fluid, and bile. Mean tissue concentrations are generally 50% to 100% of those in plasma. Distribution of Piperacillin and Tazob8ctam into cerebrospinal fluid is low in subjects with non-inflamed meninges, as with other Penicillins.
Piperacil!in is metabolized to a minor microbiologically active desethyl metabolite. Tazobactam is metabolized to a single metabolite that lacks pharmacological and antibacteirial activities. Both Piperacillin and Tazobactam are eliminated via the kidney by glomerular filtration and tubular secretion. Piperacillin is excreted rapidly as unchanged drug with 68% of the administered dose excreted in the urine. Tazobactam and its metabolite are eliminated primarily by renal excretion with 80% of the administered dose excreted as unchanged drug and the remainder as the single metabolite. Piperacillin, Tazobactam and desethy1 PiperaciUin are also secreted into the bile.
Dosage and Administration:
Piperacillin and Tazobactam for injection are administered by intravenous infusion over 30 minutes. The usual total daily dose of Piperacillin and Tazobactam for injection for adults is 3.375 g every six hours totaling 13.5 g (12.0 g Piperacillin/1.5 g Tazobactam). Or as prescribed by the physician.
Direction and Reconstitution:
2.25 g, 3.375 g, and 4.5 g Piperacillin and Tazobactam for Injection should be reconstituted with 10 ml, 15 ml and 20 ml respectively. Swirl until dissolved.
For conventional vials, reconstitute Piperacillin and Tazobactam for lnjection per gram of Piperacillin with 5 ml of reconstitution · diluent. Shake well until dissolved. • Discard any unused portion.
Precautions
Bleeding manifestations have occurred in some patients receiving beta-lactam antibiotics, including Piperacillin. These reactions have sometimes been associated with abnormalities of coaQulation tests such as clotting time, platet aggregation and prothrombin time, and are more likely to occur in patients with renal failure. If bleeding manifestations occur, Piperacillin and Tazobactam for injection should be discontinued and appropriate therapy instituted. The possibility of the emergence of resistant organisms that might cause superinfections should be kept in mind. lf this occurs, appropriate measures should betaken.
Piperacillin is excreted in low concentrations in human milk; Tazobactam concentrations in human milk have not been studied. Caution should be exercised when PiperaciHin and Tazobactam for injection is administered to a nursing woman.
Pregnancy and Lactation
Certain medicines should not be used during pregnancy or breast-feeding. However, other medicines may be safety used in pregnancy or breast-feeding providing the benefits to the mother outweigh the risks to the unborn baby. Always inform your doctor if you are pregnant or planning a pregnancy, before using any medicine.
The safety of this medicine during pregnancy has not been fully established. The manufacturer recommends it should ooly be used on pregnant women if the expected benefit to the mother is greater than any possible risk to the developing fetus. Seek medical advice from your doctor. This medicine passes into breast milk in small amounts. The manufacturer recommends it should only be used in breast feeding mothers if the expected benefit to the mother is greater than any possible risk to the nursing infant. Seek medical advice from your doctor.
Drug Interactions:
Probenecid
Probenecid administered concomitantly with Piperacillin and Tazobactam for injection the prolongs half-life of Piperacillin-by 21 % and that ofTazobactam by 71 %.
Vancomycin
No pharmacokinetic interactions have been noted ·between Piperacillin and Tazobactam for injection and Vancomycin.
Heparin
Coagulation parameters should be tested more frequently and monitored regularly during simultaneous administration of high doses of Heparin, oral anticoagulants, or other drugs that may affect the blood coagulation system or the thrombocyte function.
Vecuronium
Piperacillin when used concomitantly with Vecuronium has been implicated in the prolongation of the neuromuscular blockade of Vecuronium. Piperacillin/Tazobactam could produce the same phenomenon if given along with Vecuronium. Due to their similar mechanisms of action, it is·expected that the neuromuscular blockade produced by any of the non-depolarizing muscle relaxants could be prolonged in thb presence of Piperacillin.
Methotrexate
Limited data suggests that co-administration of Methotrexate and Piperacitlin may reduce the clearance of Methotrexate due to competition for renal secretion. The impact ofTazof>actam on the elimination of Methotrexate has not been evaluated. If concurrent therapy is necessary, serum concentrations of Methotrexate as well as the signs and symptoms of Methotrexate’t6xicity should be frequently monitored.
Side Effects:
Piperacillin and Tazobactam may cause side effects s4.ch as upset stomach, vomiting, unpleasant or abnormal taste, diarrhea, gas, head?tche, constipation, insomnia, rash, itching, and skin swelling, shortness of breath, unusual bruising or bleeding.
Storage:
Store at temperatures not exceeding 30°C. Keep out f reach of children. Protect from light.
Specification:
ln house
Caution:
Foods, Drugs, Devices and Cosmetics Act prohibits dispensing without prescription.
Availability:
Box of 1 vial + (2) 10 ml ampoule Water for Injection as diluent
SHELF LIFE: 36 months
References: Martindale 33″‘ Edition. The Complete Drug Reference Martindale 34″‘ Edition. The,Complete Drug Reference
For suspected adverse drug reaction report to the FDA: www.fda.gov.ph
Seek medical attention immediately at the first sign of any adverse drug reaction.
DRP-3295
Date of leaflet revision: APR 2017
location_on Lagazo Village, Calinan, Davao City
local_phone (082) 299 1768
phone_android +63 917 301 4186