

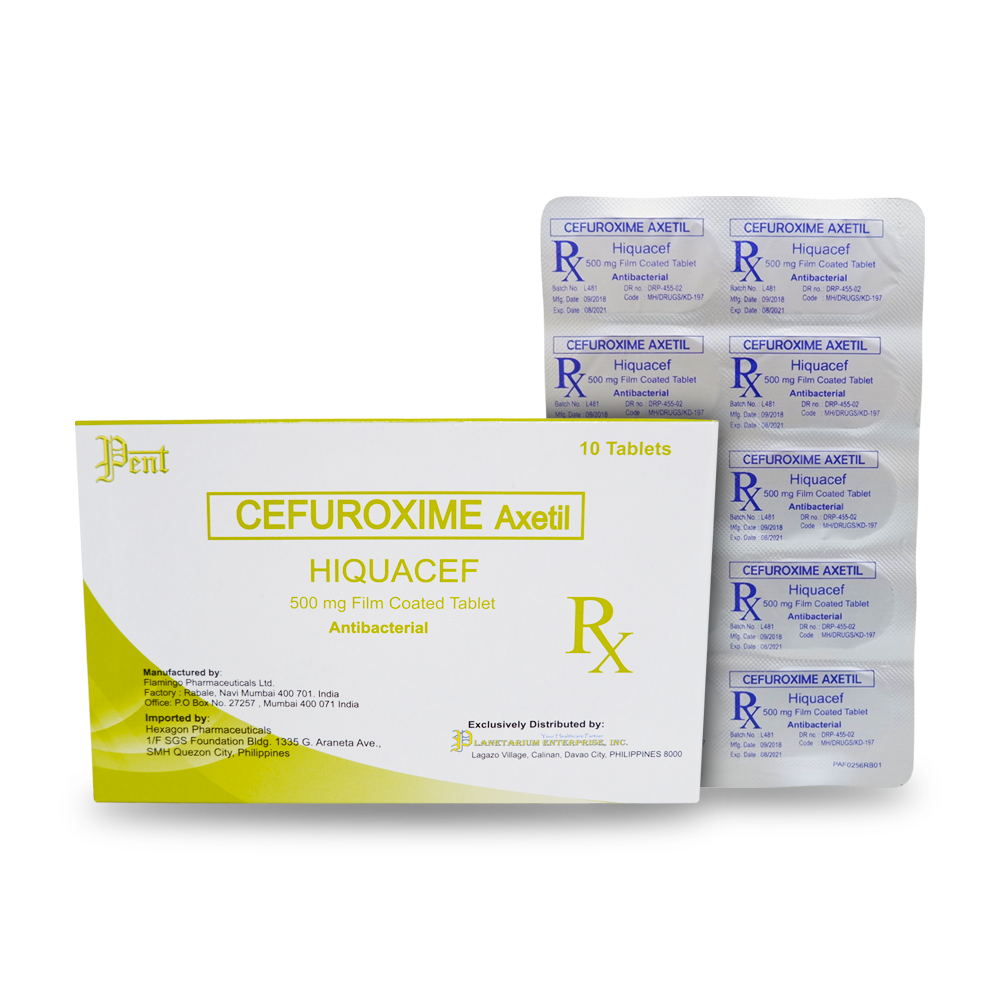
Generic Name: CEFUROXIME AXETIL
Hiquacef 500mg
Film-coated tablet
ANTIBACTERIAL
Formulation:
Each film coated tablet contains:
Cefuroxime (as axetil) USP 500mg
Mechanism of Action:
The in vivo bactericidal activity of Cefuroxime axetil is due to cefuroxime’s binding to essential target proteins and the resultant inhibition of cell-wall
synthesis. Cefuroxime has bactericidal activitiy against a wide range of common pathogens, including many beta-lactamase producing strains. Cefuroxime is stable to many bacterial beta-lactamases, especially plasmid-mediated enzymes that are commonly found in enterobacteriaceae.
Pharmacokinetics:
Absorption and Metabolism After oral administration, Cefuroxime axetil is absorbed from the gastrointestinal tract and rapidly hydrolyzed by nonspecific esterases in the intestinal mucosa and blood to cefuroxime. Cefuroxime is subsequently distributed throughout the extracellular fluids. The axetil moeity is metabolized to acetaldehyde and acetic acid. Distribution Approximately 50% of serum cefuroxime is bound to protein. Food Effect On Pharmacokinetics
Cefuroxime is excreted unchanged in the urine; in adults, approximately 50% of the administered dose is recovered in the urine within 12 hours. Because cefuroxime is renally excreted, the serum half-life is prolonged in patients with reduced renal function.
Indications:
Cefuroxime axetil is indicated for the treatment of the following mild to moderately severe infections in adults caused by sensitive bacteria.
Acute upper respiratory infections:
otitis media, sinusitis, tonsillitis and pharyngitis.
Acute exacerbations of chronic bronchitis, or acute bronchitis.
Skin and skin structure infections for example, furunculosis, pyoderma and impetigo.
Acute uncomplicated gonococcal urethritis, and cervicitis due to non-penicillinase producing gonococci.
Dosage and Administration:
The usual course of therapy with Cefuroxime axetil tablets is 5 to 7 days for treatment of bronchitis, and 7 to 10 days for other infections. Cefuroxime axetil should be taken after a light meal for optimum absorption.
Adults: Acute exacerbations of chronic bronchitis- 250mg to 500mg twice daily. Acute bronchitis – 250mg to 50mg twice daily
Uncomplicated gonococcal urethritis or cervicitis – single dose of 1g
Other infections – 250mg twice daily
Or as prescribed by the physician.
Overdosage:
Overdosage can cause cerebral irritation leading to convulsions. Serum levels of cefuroxime can be reduced by hemodialysis and peritoneal dialysis.
Contraindications:
Cefuroxime axetil products are contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
Precautions:
General
As with other broad-spectrum antibiotics, prolonged administration of Cefuroxime axetil may result in overgrowth of nonsusceptible microorganisms. If superinfection occurs during theraphy, appropriate measures should be taken. Cephalosporins, including Cefuroxime axetil, should be given with caution to patients receiving concurrent treatment with potent diuretics because these diuretics are suspected of adversely affecting renal function. Cefuroxime axetil, as with other broad-spectrum antibiotics, should be prescribed with caution in individuals with history of colitis. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
Warnings:
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including cefuroxime, and may range from mild to life threatening. Treatment with antibacterial agents alters normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of antibiotic-associated colitis. After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug effective against Clostridium difficile.
Pregnancy, Teratogenic Effects, Pregnancy Category B:
Reproduction studies have been performed in rats and mice at doses up to 3200 mg/kg per day (23 times the recommended human dose based on mg/m²) and have revealed no evidence of harm to the fetus due to Cefuroxime axetil. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Drug Interactions:
Concomitant administration of probenecid with cefuroxime axetil increases the area under the serum concentration versus time curve by 50%. Drugs that reduce gastric acidity may result in a lower bioavailability of cefuroxime axetil compared with that of fasting state and tend to cancel the effect of post prandial absorption.
Adverse reactions:
General:
The following hypersensitivity reactions have been reported: Anaphylaxis, angioedema, pruritus, serum-sickness-like reaction, and urticaria.
Gastrointestinal: Pseudomembranous colitis.
Hematologic: Hemolytic anemia, leukopenia, pantocytopenia, thrombocytopenia and increased prothrombin time.
Hepatobiliary Tract and Pancreas: Hepatic impairment including hepatitis and cholestasis jaundice.
Neurologic: Seizure
Skin: Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis.
Renal: Renal dysfunction.
Cephalosporin-Class Adverse Reactions:
In addition to the adverse reactions listed above that have been observed in patients treated with Cefuroxime axetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics: renal dysfunction, toxic nephropathy, heoatic cholestasis, aplastic anemia, hemolytic anemia, hemorrhage, increased prothrombin time, increased BUN, increased creatinine clearance, false-positive test for urinary glucose, increased alkaline phosphatase, neutropenia, thrombocytopenia, leukopenia, elevated bilirubin, pancytopenia and agranulocytosis.
Storage: Store at a temperature not exceeding 30°C. Protect from light. Keep out reach of children.
Caution: Foods, Drugs, Devices and Cosmetics Act Prohibits dispensing without prescription.
Availability: Alu-alu pack of 10 tablets/Box of 10’s.
For suspected adverse drug reaction, report to the FDA: www.fda.gov.ph
location_on Lagazo Village, Calinan, Davao City
local_phone (082) 299 1768
phone_android +63 917 301 4186